What does the Biopharmaceutical industry include?
Biopharmaceuticals are either medical products with biological sources, or are produced by bioprocessing techniques, such as gene and protein expression. Typical biopharmaceuticals include:
- monoclonal antibodies
- Vaccines
- Hormones
- Enzymes
- Peptides
- Stem cell therapy
- Gene therapy
- Immunotherapy
Flow chat
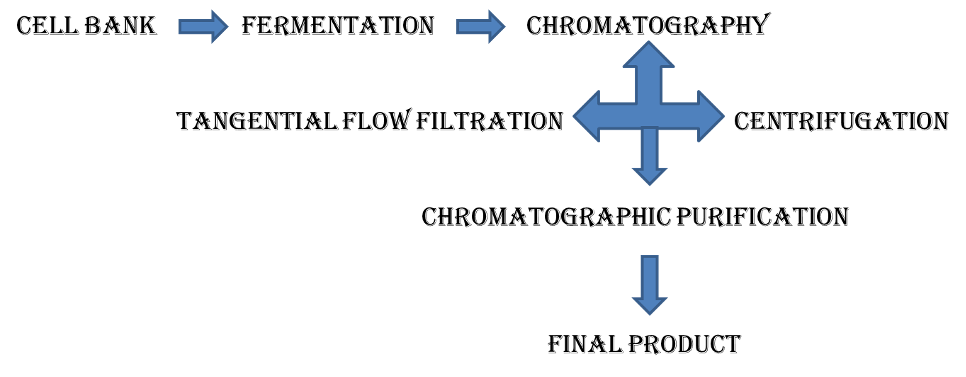
Biopharmaceutical development
The development of biopharmaceutical products has a significantly high failure rate and typical pressure-points include longer and more stringent clinical trials and a slow technology transfer to scale up the manufacturing process. The intrinsic variability in biopharmaceuticals (including the variability in the tests used for analysis) adds an additional level of complexity in justifying and defining specifications.
The registration of biopharmaceuticals and their governing bodies
Biopharmaceuticals are subject to the same process of registration as pharmaceutical products. For rDNA technology, controlled expression of genes, hybridomas, and monoclonal antibodies, a Central Drugs Standard Control Organization (CDSCO) Application is mandatory in India.
International Regulatory Agencies
The regulatory bodies responsible for governing biopharmaceuticals are the same as for pharmaceutical products and include both National and regional organizations such as the Food and Drug Administration (FDA) in the US, the Medicine and Healthcare Product Regulatory Agency (MHRA) in the UK, and the European Medicines Agency (EMA). There are some additional specialist agencies such as the FDA’s Centre for Biologics Evaluation and Research (CBER) and the National Institute for Biological Standards and Controls (NIBSC) in the UK that are dedicated to the regulation and approval of biological products.
How Gnanika TechBio can help?
Gnanika TechBio was founded on the industry’s need for specialized sector knowledge to navigate the highly detailed regulations and guidance around biopharmaceuticals. The nature of biopharmaceuticals means that operators in this area need highly specialized training and expertise gained over many years. For this reason, it is generally not considered possible to transition pharmaceutical regulations to biopharmaceuticals.
Gnanika TechBio has experience in supporting the development of biopharmaceutical products, including process development, validation, comparability/biochemical bridging, nonclinical and clinical development, agency interactions, and submission and maintenance of Product Licenses for several biopharmaceuticals and vaccines throughout India and the US.
Gnanika TechBio has supported a number of biologics manufacturers through a flexible resourcing solution to address immediate and long-term strategy, regulatory, and quality needs.

With access to
GNANIKA TECHBIO
PVT LTD
Assistance
Schedule your appointment Now